Products
Lifecosm SARS-CoV-2 & Influenza A/B Antigen Combo Test Cassette
Introduction
| Summary | Detection of specific Antigen of Covid-19 & Influenza A/B within 15 minutes |
| Principle | One-step immunochromatographic assay |
| Detection Targets | Covid-19 & Influenza A/B Antigen |
| Sample | Nasopharyngeal Swab,Oropharyngeal Swab |
| Reading time | 10~ 15 minutes |
| Quantity | 1 box (kit) = 25 devices (Individual packing) |
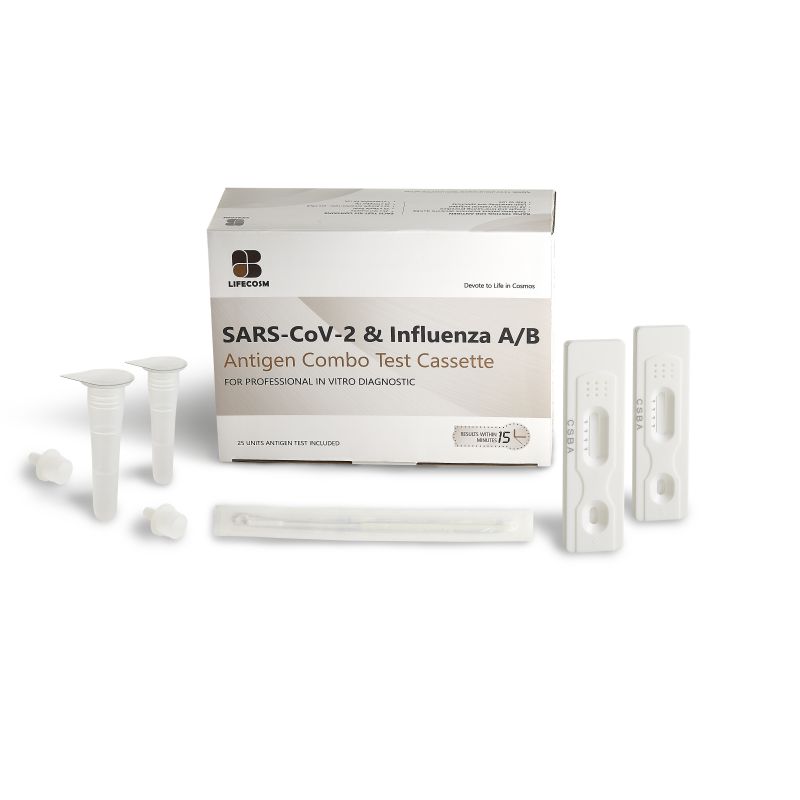
| Contents | 25 Test Cassettes: each cassette with desiccant in individual foil pouch 25 Sterilized Swabs: single use swab for specimen collection
25 Extraction Tubes: containing 0.4mL of extraction reagent 25 Dropper Tips 1 Work Station 1 Package Insert |
|
Caution |
Use within 10 minutes after openingUse appropriate amount of sample (0.1 ml of a dropper)
Use after 15~30 minutes at RT if they are stored under cold circumstances Consider the test results as invalid after 10 minutes |
INTENDED USE
The SARS-CoV-2 & Influenza A/B Antigen Combo Test Cassette is applicable to the simultaneous qualitative detection and differentiation of novel Coronavirus (SARS-CoV-2 Antigen), Influenza A virus, and/or Influenza B virus Antigen in population Oropharyngeal swabs and Nasopharyngeal swabs samples in vitro.
PRINCIPLE
The SARS-CoV-2 & Influenza A/B Antigen is qualitatively detected in population Oropharyngeal swabs and Nasopharyngeal swabs samples by the colloidal gold method. After sample added, the SARS-CoV-2 Antigen (or Influenza A/B) in the sample to be tested is combined with the SARS-CoV-2 Antigen (or Influenza A/B) antibody labelled with colloidal gold on the binding pad to form the SARS-CoV-2 Antigen (or Influenza A/B) antibody-colloidal gold complex. Due to chromatography, the SARS-CoV-2 Antigen (or Influenza A/B)-antibody-colloidal gold complex diffuses along the nitrocellulose’s membrane. Within the detection line area, the SARS-CoV-2 Antigen (or Influenza A/B)-antibody complex binds to the antibody enclosed within the detection line area, showing a purple-red band. Colloidal gold labelled SARS-CoV-2 Antigen (or Influenza A/B) antibody diffuses to the quality control line (C) region and is captured by sheep anti-mouse IgG to form red bands. When the reaction is over, the results can be interpreted by visual observation.
COMPOSITION
Materials Provided
●25 Test Cassettes: each cassette with desiccant in individual foil pouch
●25 Sterilized Swabs: single use swab for specimen collection
●25 Extraction Tubes: containing 0.4mL of extraction reagent
●25 Dropper Tips
●1 SARS-CoV-2 antigen positive control swab (optional)
●1 flu A antigen positive control swab (optional)
●1 flu B antigen positive control swab (optional)
●1 negative control swab (optional)
●1 Work Station
●1 Package Insert
Materials Required but not Provided
●Timer